The ePatch electric bandage accelerates healing
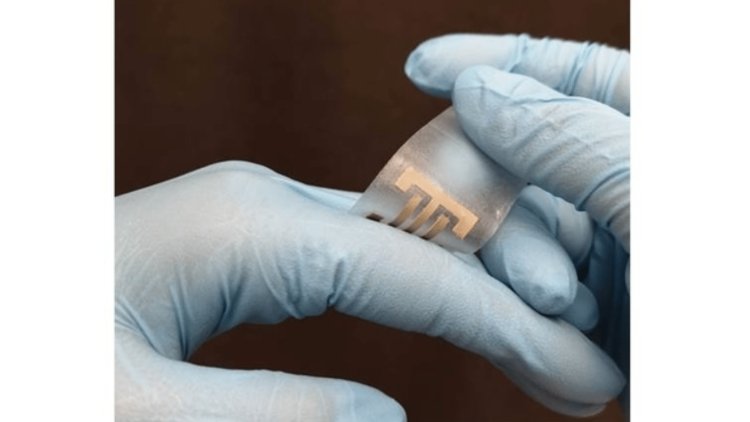
It has been known for some time that electricity can help heal wounds. The new experimental ePatch dressing uses this approach and, in addition, supports the healing process by killing bacteria.
The ePatch was developed at the Terasaki Institute for Biomedical Innovation in Los Angeles and contains electrodes made of silver nanowires mixed into a seaweed-derived hydrogel known as alginate. It is already used in surgical dressings because it is biocompatible and maintains optimal moisture levels.
By chemically modifying alginate and adding calcium to it, scientists have been able to increase the functionality and stability of silver nanowires. The resulting hydrogel was extruded onto a flexible silicone sheet, the surface of which was partially covered with a membrane-like stencil.
When this template was subsequently removed, the remaining alginate formed two electrodes. These were then firmly connected to an external power source.
Due to the different sizes and shapes of silicone foils, it was possible to create patches capable of covering a wide variety of wounds and adapting to their contours.
When testing this technology on rats with external wounds, it was found that the supplied electric current accelerates healing not only by causing the migration of skin and other granulation cells to the site but also by causing the formation of blood vessels and reducing inflammation.
While the wounds of the untreated control group healed for 20 days, the wounds of the ePatch-treated rats healed in only seven days. In addition, due to the antibacterial properties of silver, the infection was kept to a minimum.
The treated rats had less scarring than the control group after the ePatch dressing was removed at the end of the healing procedure. This could be because the skin cells do not adhere to the silicon substrate and so do not rip when the dressing is removed.
Also, an interesting topic - Researchers at Boston University have developed small mechanical hearts powered by real heart cells that can be used in experiments.
Its exact name is cardiac miniaturized Precision-enabled Unidirectional Microfluidic Pump, abbreviated miniPUMP, and has an area of only 3 cm2. It replicates the function of the real heart chamber and pumps water in the same way that the heart pumps blood.
The device consists of a plastic base on which are placed miniature acrylic valves made by 3D printing, a tube, and the pump itself. It contains a scaffold consisting of a series of interconnected concentric acrylic spirals, populated with living human cardiomyocytes (heart muscle cells). As these cells expand and contract, the flexible scaffold material moves with them and pumps water through the miniPUMP.
Cardiomyocytes can be produced from skin cells, blood cells, or nearly any other readily available cell type. These are reprogrammed to become stem cells, which then develop into heart cells.
This means that patients could have miniPUMPs made from their own cells to find out, for example, how their hearts will respond to different medicines. The same technology could one day be used to make other organ-on-chip devices, such as the lungs and kidneys.




























